Some years ago, my daughter came back from religions school and asked for a definition of science. I told her that science was the opposite of religion. I didn’t mean to insult religion or science; the big bang for one thing, strongly suggests there is a God -creator, and quantum mechanics suggests (to me) that there is a God -maintainer, but religion deals with other things beyond a belief in God, and I meant to point out that every basic of how science looks at things finds its opposite in religion.
Science is based on reproducibility and lack of meaning: if you do the same experiment over and over, you’ll always get the same result as you did before and the same result as anyone else — when the results are measured to some good, statistical norm. The meaning for the observation? that’s a meaningless question. Religion is based on the centrality of drawing meaning, and the centrality of non-reproducible, one-time events: creation, the exodus from Egypt, the resurrection of Jesus, the birth of Zeus, etc. A religious believer is one who changes his or her life based on the lesson of these; to him, a non-believer is one who draws no meaning, or needs reproducible events.
Science also requires that anyone will get the same result if they do the same process. Thus, chemistry class results don’t depend on the age, sex, or election of the students. Any student who mixes the prescribed two chemicals and heats to a certain temperature is expected to get the same result. The same applies to measures of the size of the universe, or its angular momentum or age. In religion, it is fundamentally important what sex you are, how old you are, who your parents were, or what you are thinking at the time. If the right person says “hoc es corpus” over wine and wafers, they change; if not, they do not. If the right person opens the door to heaven, or closes it, it matters in religion.
A main aspect of all religion is prayer; the idea that what you are thinking or saying changes things on high and here below. In science, we only consider experiments where the words said over the experiment have no effect. Another aspect of religion is tchuvah (regret, repentance); the idea is that thoughts can change the effect of actions, at least retroactively. Science tends to ignore repentance, because they lack the ability to measure things that work backwards in time, and because the scientific instruments we have currently do not take measurements on the soul to see if the repentance had any effect. Basically, the science-universe is only populated with those things which can be measured or reproducibly affected, and that pretty much excludes the soul. That the soul does not exist in the science universe doesn’t mean it doesn’t exist.
Another main aspect of religion is morality: you’re supposed to do the right thing. Morality varies from one religion to another, and you may think the other fellow’s religion has a warped morality, but at least there is one in all religions. In science, for better or worse, there is no apparent morality, either to man or to the universe. Based on science, the universe will end, either by a bang or a whimper, and in that void of end it would seem that killing a mouse is about as important as killing a person. No religion I know of sees the universe ending in either cold or hot death; as a result. Consistent with this, they all see murder is a sin against God. This difference is a big plus for religion, IMHO. That man sees murder as a true evil is either a sign that religion is true, or that it isn’t depending on the value you put on life. Another example of the moral divide: Scientists, especially academics, tend to be elitists. Their morality, such as it is, values great minds and great projects over the humble and stupid. Classical religion sees the opposite; it promoting the elevation of the poor, weak, and humble. There is no fundamental way to tell which one is right, and I tend to think that both are right in their own, mirror-image universes.
It is now worthwhile to consider what each universe sees as wisdom. An Explanation in the universe of science has everything to do with utility and not any internal sense of having understood, as such. I understand something only to the extent I predict that thing or can do something based on the knowledge. in religion, the motivation for all activity is always just understanding — typically of God on the bone-deep level. This difference shows up very clearly in dealing with quantum mechanics. To a scientist, the quantum world is fundamentally a door from religion because it is basically non-understandable but very useful. Religion totally ignores quantum mechanics for the same reason: it’s non-understandable, but very precise and useful. Anything you can’t understand is meaningless to them (literally), and useful is mostly defined in terms of building the particular religion; I think this is a mistake on many levels. I note that looking for disproof is the glory-work of all science development, but the devil’s work of every religion. A religious leader will grab on to statistical findings that suggest that his type of prayer cures people, but will always reject disproof, e.g. evidence that someone else’s prayers works better, or that his prayer does nothing at all. Each religion is thus in a war with the other, each trying to build belief, while not removing it. Science is the opposite. Religion starts with the answer and accepts any support it can; fundamental change is considered a bad thing in religion. The opposite is so with science; disproof is considered “progress,” and change is good.
These are not minor aspects of science and religion, by the way, but these are the fundamental basics of each, as best I can tell. History, politics, and psychology seem to be border-line areas, somewhere between science and religion. The differences do not reflect a lack in these fields, but just a recognition that each works according to its own logic and universe.
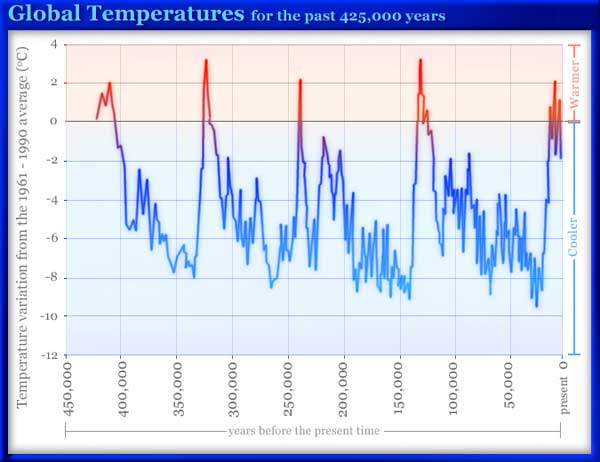
My hope in life is to combine science and religion to the extent possible, but find that supporting science in any form presents difficulties when I have to speak to others in the religious community, my daughter’s teachers among them. As an example of the problem that come up, my sense is that the big bang is a fine proof of creation and should be welcomed by all (most) religious people. I think its a sign that there is a creator when science says everything came from nothing, 14,000,000 years ago. Sorry to say, the religious leaders I’ve met reject the big bang, and claim you can’t believe in anything that happened 14,000,000,000 years ago. So long as science shows no evidence of a bearded observer at the center, they are not interested. Scientists, too have trouble with the bang, I find. It’s a one-time event that they can’t quite explain away (Steven Hawking keeps trying). The only sane approach I’ve found is to keep blogging, and otherwise leave each to its area. There seems to be little reason to expect communal agreement.
by Robert E. Buxbaum, Apr. 7, 2013. For some further thoughts, see here.