The first thing to know about sewage is that it’s mostly water and only about 250 ppm solids. That is, if you boiled down a pot of sewage, only about 1/40 of 1% of it would remain as solids at the bottom of the pot. There would be some dried poop, some bits of lint and soap, the remains of potato peelings… Mostly, the sewage is water, and mostly it would have boiled away. The second thing to know, is that the solids, the bio-solids, are a lot like soil but better: more valuable, brown gold if used right. While our county mostly burns and landfills the solids remnant of our treated sewage, the wiser choice would be to convert it to fertilizer. Here is a comparison between the composition of soil and bio-solids.

The composition of soil and the composition of bio-solid waste. biosolids are like soil, just better.
Most of Oakland’s sewage goes to Detroit where they mostly dry and burn it, and land fill the rest. These processes are expensive and engineering- problematic. It takes a lot of energy to dry these solids to the point where they burn (they’re like really wet wood), and even then they don’t burn nicely. As shown above, the biosolids contain lots of sulfur and that makes combustion smelly. They also contain nitrate, and that makes combustion dangerous. It’s sort of like burning natural gun powder.
The preferred solution is partial combustion (oxidation) at room temperature by bacteria followed by conversion to fertilizer. In Detroit we do this first stage of treatment, the slow partial combustion by bacteria. Consider glucose, a typical carbohydrate,
-HCOH- + O2 –> CO2 + H2O. ∆G°= -114.6 kcal/mol.
The value of ∆G°, is relevant as a determinate of whether the reaction will proceed. A negative value of ∆G°, as above, indicates that the reaction can progress substantially to completion at standard conditions of 25°C and 1 atm pressure. In a sewage plant, many different carbohydrates are treated by many different bacteria (amoebae, paramnesia, and lactobacilli), and the temperature is slightly cooler than room, about 10-15°C, but this value of ∆G° suggests that near total biological oxidation is possible.
The Detroit plant, like most others, do this biological oxidation treatment using either large stirred tanks, of million gallon volume or so, or in flow reactors with a large fraction of cellular-material returning as recycle. Recycle is needed also in the stirred tank process because of the low solid content. The reaction is approximately first order in oxygen, carbohydrate, and bacteria. Thus a 50% cell recycle more or less doubles the speed of the reaction. Air is typically bubbled through the reactor to provide the oxygen, but in Detroit, pure oxygen is used. About half the organic carbon is oxidized and the remainder is sent to a settling pond. The decant (top) water is sent for “polishing” and dumped in the river, while the goop (the bottom) is currently dried for burning or carted off for landfill. The Holly, MI sewage plant uses a heterogeneous reactors for the oxidation: a trickle bed followed by a rotating disk contractor. These have higher bio-content and thus lower area demands and separation costs, but there is a somewhat higher capital cost.
A major component of bio-solids is nitrogen. Much of this in enters the form of urea, NH2-CO-NH2. In an oxidizing environment, bacteria turns the urea and other nitrogen compounds into nitrate. Consider the reaction the presence of washing soda, Na2CO3. The urea is turned into nitrate, a product suitable for gun powder manufacture. The value of ∆G° is negative, and the reaction is highly favorable.
NH2-CO-NH2 + Na2CO3 + 4 O2 –> 2 Na(NO3) + 2 CO2 + 2 H2O. ∆G° = -177.5 kcal/mol
The mixture of nitrates and dry bio-solids is highly flammable, and there was recently a fire in the Detroit biosolids dryer. If we wished to make fertilizer, we’d probably want to replace the drier with a further stage of bio-treatment. In Wisconsin, and on a smaller scale in Oakland MI, biosolids are treated by higher temperature (thermophilic) bacteria in the absence of air, that is anaerobically. Anaerobic digestion produces hydrogen and methane, and produces highly useful forms of organic carbon.
2 (-HCOH-) –> CO2 + CH4 ∆G° = -33.7 Kcal/mol
3 (-HCOH-) + H2O –> -CH2COOH + CO2 + 2 1/2 H2 ∆G° = -21.9 kcal/mol
In a well-designed plant, the methane is recovered to provide heat to the plant, and sometimes to generate power. In Wisconsin, enough methane is produced to cook the fertilizer to sterilization. The product is called “Milorganite” as much of it comes from Milwaukee and much of the nitrate is bound to organics.

Egg-shaped, anaerobic biosolid digestors, Singapore.
The hydrogen could be recovered too, but typically reacts further within the anaerobic digester. Typically it will reduce the iron oxide in the biosolids from the brown, ferric form, Fe2O3, to black FeO. In a reducing atmosphere,
Fe2O3 + H2 –> 2 FeO + H2O.
Fe2O3 is the reason leaves turn brown in the fall and is the reason that most poop is brown. FeO is the reason that composted soil is typically black. You’ll notice that swamps are filled with black goo, that’s because of a lack of oxygen at the bottom. Sulphate and phosphorous can be bound to ferrous iron and this is good for fertilizer. Generally you want the reduction reactions to go no further.

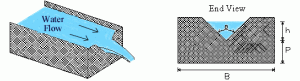
Weir dam on the river Dour in Scotland. Dams of this type increase residence time, and oxygenate the flow. They’re good for fish, pollution, and flooding.
When allowed to continue, the hydrogen produced by anaerobic digestion begins to reduce sulfate to H2S.
NaSO4 + 4.5 H2 –> NaOH + 3H2O + H2S.
I’m running for Oakland county, MI water commissioner, and one of my aims is to stop wasting our biosolids. Oakland produces nearly 1000,000 pounds of dry biosolids per day. This is either a blessing or a curse depending on how we use it.
Another issue, Oakland county dumps unpasteurized, smelly black goo into Lake St. Clair every other week, whenever it rains more than one inch. I’d like to stop this by separating the storm and “sanitary” sewage. There is a capital cost, but it can save money because we’d no longer have to pay to treat our rainwater at the Detroit sewage plant. To clean the storm runoff, I’d use mini wetlands and weir dams to increase residence time and provide oxygen. Done right, it would look beautiful and would avoid the flash floods. It should also bring natural fish back to the Clinton River.
Robert Buxbaum, May 24 – Sept. 15, 2016 Thermodynamics plays a big role in my posts. You can show that, when the global ∆G is negative, there is an increase in the entropy of the universe.
Like this:
Like Loading...