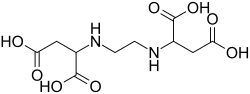
Among the products our company sells is a non-toxic chelating agent, EDDS (ethylenediamine-disuccinic acid), typically sold as a purified salt in ammonia solution, see here. The main use of EDDS is to stabilize heavy metal ions in solution. We use it, for example, as an aide in electroless Palladium coating, to stabilize palladium ions, helping us produce a smaller grain, more continuous coat. The structure, shown below, is similar to that of EDTA (ethylenediaminetetraacetic acid), and the behavior is similar too. EDDS is more stabilizing in the presence of the other ions and we like that it is non-toxic.
The popular literature use for chelating agents like this is as a treatment for heavy metal poisoning by lead, arsenic, cadmium, nickel or copper. The TV series “House” featured patients with all these metal-poisoning problems, problems. Chelation treatment was important in Flint Michigan, 2015 when thousands got low-level lead poisoning and legionaries disease after the water department put insufficient phosphate and hypochlorite into the water and lead leached from pipes. Typically, EDTA is used for humans here, while EDDS is used by farmers and ranchers to treat animals. EDDS is less toxic, and removes fewer essential light minerals: magnesium, calcium, and zinc, so I’d think it would be better for humans too.
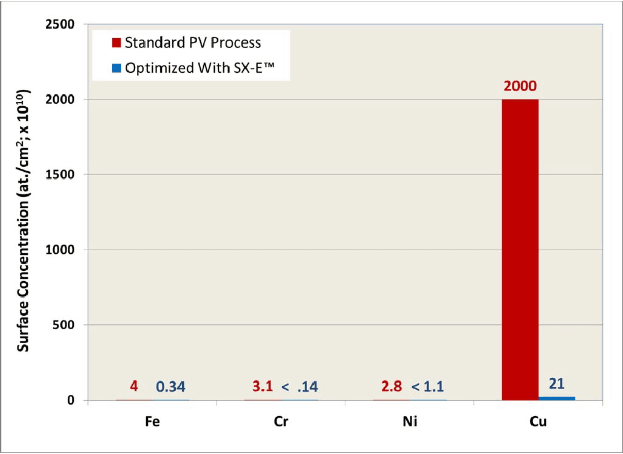
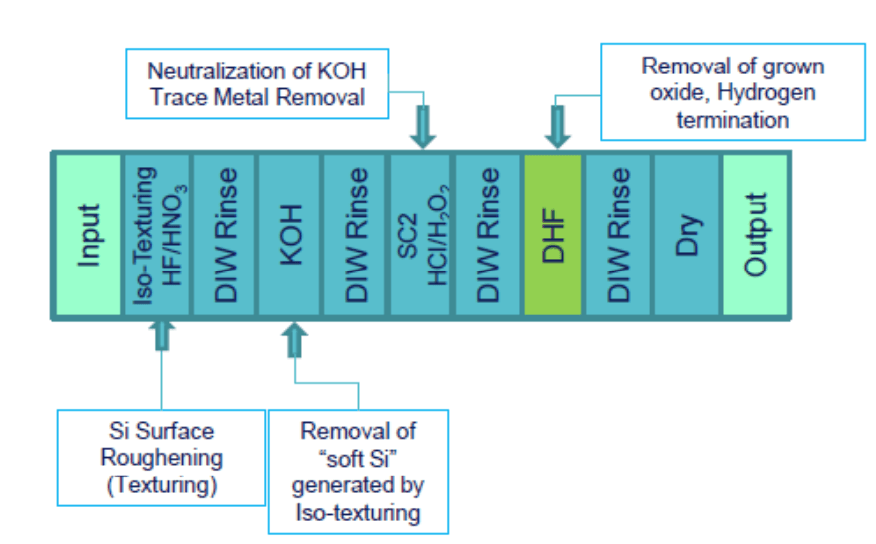
Our EDDS has been used to make cleaning solutions for silicon wafers, Sunsonix SXE, for example. Sunsonix SXE behaves as a soap, removing Fe, Cr, Ni, and Cu from solar cells, see reproduced figures at right. These metals will diffuse into surface of a silicon wafer, forming defects that absorb light and decrease solar cell performance by an average of 0.28%, see below.
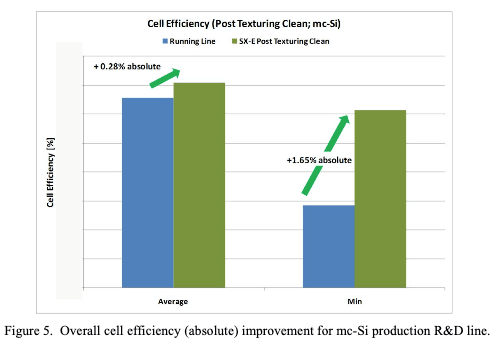
This is, based on a baseline efficiency of 16%. For more details see “Surface Contamination Removal from Si PV Substrates Using a Biodegradable Chelating Agent and Detection of Cleaning Endpoints Using UV/VIS Spectroscopy” ECS Transactions, 41 (5) 295-302 (2011). See also this article in Wikipedia.
At a different pH, EDDS and EDTH are used in remediation of metal-contaminated soils, see here. This can be done ex-situ, with the soil taken out to an external site and then washed. Alternately, for less contaminated soils, remediation can be done in-situ with the chelating wash applied to the soil. Plants, like vetiver grass (Chrysopogon zizanioides) then extract the heavy metals, concentrating them in their leaves. EDDS is more suitable for this as it is biodegradable and shows a high extraction efficiency in mineral rich soils, see here for comparison to EDTA.
Moving to another area of extraction. It seems that EDDS or EDTA solutions can be used to profitably extract rare earth metals, perhaps sending them to plants before final concentration. A standard methods of rare earth extraction uses chlorine and high temperatures. Alternate methods use ion-exchange extraction of liquid-liquid extraction. I suspect that chelation treatment might turn out to be more effective and cheaper. The price of rare earths has risen in recent years as China restricts sales so that the need for a new source has become a national priority.
Robert Buxbaum, January 13, 2026