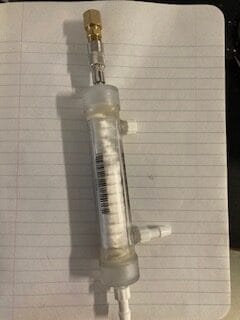
Generally, when you make hydrogen, you make wet hydrogen, hydrogen contaminated with water. Usually you want to dry the hydrogen before you use it or compress it. if you compress the hydrogen for transport or storage without drying it, the water will condense and perhaps freeze, clogging valves and fittings.
Water contamination of hydrogen is also a problem for brazing. Hydrogen is a good, cover gas for brazing because of its high heat transfer properties and its reducing chemistry. When the hydrogen is contaminated with water vapor it is unstable for use with stainless steel and similar metals as it will cause oxidation of the surface, resulting in a grey-green surface, and preventing good brazing. Some other contaminates can be problems, e.g. CO2 but water is the main problem in brazing environments.
One more example where drying hydrogen is important, is for its use in high altitude balloons. At high altitudes, water can condense, changing the lift characteristics, and perhaps freezing and puncturing the balloon. For all these applications, I suggest use of a silicone polymeric membrane operated as dryers, using a counter current flow as shown below. We sell these at REB Research, see here. These membranes also remove CO2, silanes, and H2S.
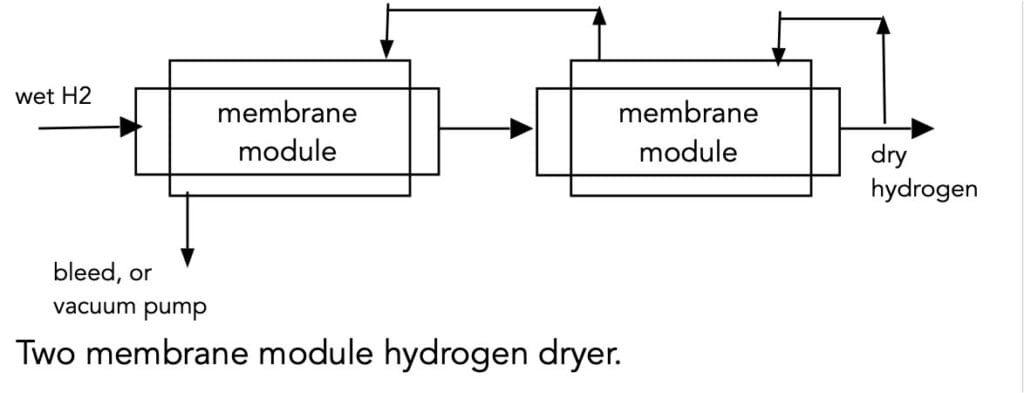
The dryer shown in the figure above has two extraction modules in series. for small flows, one module will suffice. As shown, wet hydrogen enters at left, typically at a slightly elevated pressure, 2-4 atm. The bleed stream must be at lower pressure. One atm will work for the bleed stream, but for efficient removal of the water and CO2, you will want mild vacuum, perhaps 1/3 atm. A small amount of dry hydrogen should be directed into the sweep stream as shown for efficient impurity removal. The amount directed to the bleed flow is large determined by the ratio of pressures and by the selectivity of the membrane. At a pressure ratio of ten, for example, you can show that you need at least a leaving bleed flow of 10% of the H2 to remove all the water in the hydrogen, leaving it perfectly dry. In practice, you’ll want a larger exit bleed flow, perhaps 15%, suggesting that you want a recycle stream of ~5% of the dry hydrogen. This will be joined by 10% more hydrogen that comes through the membrane modules. The membranes are 30x more selective to water than to hydrogen.
Our silicone membranes remove CO2 too, but not with as high a selectivity. For mobile use, you might want to power the vacuum pump by a fuel cell that runs on the waste, wet hydrogen of the bleed stream.
For many applications you need to remove all the impurities, including all the nitrogen and CO2. This is true for diamond making, semiconductors, and nuclear fusion. For this, you want a metallic membrane, e.g. palladium-silver. We sell hydrogen purifiers based on palladium-silver membranes for these applications. Palladium-silver membranes remove all impurities, see why here. You still need a bleed flow, but it can be much lower than the pressure ratio because, with metallic membranes, the hydrogen goes through the membrane, and the impurities stay behind. Of course, palladium costs more than plastics. See our products at www.rebresearch.com.
Robert Buxbaum, December 19, 2025